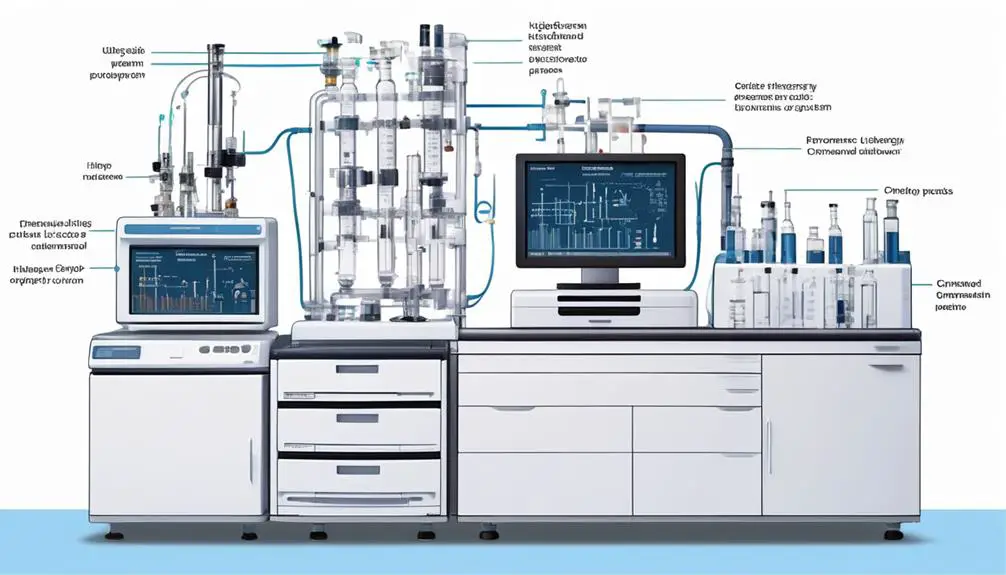
Are you curious about the inner workings of High Performance Liquid Chromatography (HPLC)? Well, get ready to uncover the principle, parts, types, uses, and even get a glimpse of a helpful diagram.
HPLC is a sophisticated analytical technique that allows for the separation and quantification of components in a sample. It does so by utilizing a mobile phase and a stationary phase, which work together to separate the components based on their unique interactions.
But that’s not all – we will also explore the various types of HPLC and their applications in fields like pharmaceutical analysis, environmental analysis, food analysis, and more.
So, buckle up and prepare to unravel the mysteries of HPLC!
HPLC Principle
The HPLC principle involves the separation of components in a sample using a mobile phase and a stationary phase. This separation is achieved by the differential interaction of the sample components with the stationary phase. The mobile phase carries the sample through the stationary phase, allowing for separation. HPLC relies on the differential retention of sample components, with some components spending more time in the stationary phase.
To perform HPLC, several key parts are required. These include a solvent reservoir for the mobile phase, a pump to deliver the mobile phase at a constant flow rate, a sample injector to introduce the sample into the mobile phase stream, a column containing the stationary phase to facilitate the separation of sample components, and a detector to detect and quantify the separated components as they elute from the column.
The HPLC technique encompasses different types, such as reverse-phase, normal-phase, ion-exchange, and size-exclusion HPLC. Reverse-phase HPLC is commonly used for separating nonpolar compounds, while normal-phase HPLC separates polar compounds using a polar stationary phase. Ion-exchange HPLC separates charged compounds based on their ionic interactions, and size-exclusion HPLC separates compounds based on their size and molecular weight.
HPLC finds applications in various fields, including pharmaceutical analysis, environmental analysis, food analysis, and forensic analysis. It’s employed for drug discovery, quality control, purity determination, impurity analysis, and compound identification. However, HPLC has limitations such as cost, sensitivity, speed, and sample throughput, which should be considered when choosing an analytical technique.
HPLC Parts
One of the key components in an HPLC system is the solvent reservoir for the mobile phase. The solvent reservoir holds the mobile phase, which is the liquid used to carry the sample through the stationary phase.
It’s important to have a solvent reservoir that’s capable of holding a sufficient amount of mobile phase for the duration of the analysis. The reservoir should be made of a material that’s compatible with the solvent being used, as some solvents may react with certain materials and affect the analysis.
Additionally, the reservoir should be equipped with a cap or lid to prevent evaporation and contamination of the mobile phase. It’s also important to ensure that the reservoir is properly sealed to maintain the desired flow rate and prevent any leaks.
The solvent reservoir is typically connected to the pump, which delivers the mobile phase at a constant flow rate.
HPLC Types
Now let’s discuss the different types of HPLC.
Reverse-phase HPLC is commonly used for separating nonpolar compounds.
Normal-phase HPLC is used for polar compounds.
Ion-exchange HPLC separates charged compounds based on their ionic interactions.
These different types of HPLC offer versatility in separating various types of compounds based on their chemical properties.
Reverse-phase HPLC
Reverse-phase HPLC, a widely used type of HPLC, separates nonpolar compounds based on their interaction with a polar stationary phase. In this technique, the stationary phase consists of a nonpolar material, such as a hydrocarbon chain bonded to silica or polymer particles.
The mobile phase, on the other hand, is a polar solvent, typically water or a mixture of water and an organic solvent. Nonpolar compounds in the sample have a higher affinity for the nonpolar stationary phase than for the polar mobile phase. As a result, they spend more time interacting with the stationary phase and elute later.
On the contrary, polar compounds elute earlier as they’ve less affinity for the nonpolar stationary phase. Reverse-phase HPLC is particularly useful for analyzing nonpolar compounds, such as hydrophobic drugs, lipids, and pesticides.
Normal-phase HPLC
Normal-phase HPLC, another type of HPLC, separates polar compounds by utilizing a polar stationary phase. In this method, the stationary phase is typically made of silica gel or alumina, which possess polar functional groups on their surface.
The polar stationary phase interacts with polar compounds in the sample, causing them to be retained longer in the column. On the other hand, nonpolar compounds pass through the column more quickly.
To elute the separated compounds, a nonpolar mobile phase is used, such as a mixture of nonpolar solvents. This creates a polarity gradient, allowing the polar compounds to be eluted from the column in order of decreasing polarity.
Normal-phase HPLC is commonly used for the separation of polar analytes, such as organic acids, carbohydrates, and some natural products.
Ion-exchange HPLC
In the realm of HPLC types, another significant technique is ion-exchange HPLC, which builds upon the principles of normal-phase HPLC.
Ion-exchange HPLC is particularly useful for separating charged compounds based on their ionic interactions. In this technique, the stationary phase consists of ion-exchange resins that contain charged functional groups. These functional groups interact with the charged analyte molecules in the sample, leading to their retention or elution.
The retention time of the analyte depends on the strength of the ionic interaction with the stationary phase. By manipulating the mobile phase composition and pH, the separation of different analytes can be achieved.
Ion-exchange HPLC is commonly used for the analysis of ionic samples, separation of anions and cations, as well as the determination of ionizable compounds in various industries, such as pharmaceutical, environmental, and food analysis.
HPLC Uses
HPLC has a wide range of applications in various industries, making it a versatile analytical technique. It’s used in pharmaceutical analysis for drug discovery, quality control, purity determination, impurity analysis, and compound identification.
HPLC is also employed in environmental analysis, food analysis, forensic analysis, and more. However, it’s important to consider the limitations of HPLC, such as cost, sensitivity, speed, and method development challenges.
Applications in Industries
HPLC finds extensive applications in various industries for the analysis, characterization, and quality control of diverse compounds and products.
In the pharmaceutical industry, HPLC is used for drug discovery, purity determination, impurity analysis, and compound identification.
It’s also employed in environmental analysis to detect and quantify pollutants.
In the food industry, HPLC is utilized to ensure the safety and quality of food products by analyzing contaminants, additives, and nutritional components.
HPLC is also valuable in forensic analysis for the identification of drugs, toxins, and other substances.
Moreover, HPLC plays a crucial role in industries such as biotechnology, chemical manufacturing, and cosmetics, where it helps in process monitoring, product development, and quality assurance.
Its versatility and accuracy make HPLC an indispensable tool in various industrial sectors.
Advantages and Limitations
Now let’s explore the advantages and limitations of utilizing HPLC for various analytical purposes.
HPLC offers several advantages, including its ability to separate a wide range of compounds, from non-polar to polar and charged species. It can handle complex mixtures and provide high resolution and sensitivity for compound analysis. HPLC is also versatile, with different types available for specific applications.
However, HPLC does have limitations. It can be costly due to the requirement of large quantities of expensive organic solvents. Some compounds may not be detectable if irreversibly adsorbed, and volatile substances are better separated by gas chromatography. HPLC also has limitations in terms of speed and sample throughput compared to other techniques. Method development and optimization can be time-consuming and challenging.
Despite these limitations, HPLC remains a valuable tool in analytical chemistry for various industries.
HPLC Diagram
The HPLC diagram illustrates the key components and their interactions in the separation process. It provides a visual representation of the flow of the mobile phase, the introduction of the sample, and the separation of components in the column. The diagram typically includes the solvent reservoir, pump, sample injector, column, and detector.
Starting from the solvent reservoir, the mobile phase is pumped through the system at a constant flow rate by the pump. The sample injector then introduces the sample into the mobile phase stream, where it’s carried through the column. The column contains the stationary phase, which interacts with the sample components to facilitate their separation.
As the sample components elute from the column, the detector detects and quantifies them.
The HPLC diagram helps to understand the different stages of the separation process and how each component plays a crucial role. It’s a useful tool for troubleshooting and optimizing HPLC methods. By analyzing the diagram, you can identify potential areas of improvement and ensure the efficiency and accuracy of the separation process.
Limitations of HPLC
After understanding the key components and interactions in the HPLC separation process, it’s important to acknowledge the limitations that come with this analytical technique.
One limitation of HPLC is its cost. HPLC requires large quantities of expensive organic solvents, making it a costly technique.
Additionally, HPLC may have low sensitivity for certain compounds, and some compounds may not be detectable if irreversibly adsorbed. It’s also worth noting that volatile substances are better separated by gas chromatography.
In terms of speed and sample throughput, HPLC has limitations compared to other analytical techniques. Method development and optimization can be time-consuming and challenging in HPLC.
Despite these limitations, HPLC remains a valuable tool in various industries for drug discovery, quality control, purity determination, impurity analysis, and compound identification. It finds applications in pharmaceutical analysis, environmental analysis, food analysis, forensic analysis, and more.
While it’s important to be aware of the limitations, HPLC’s strengths and versatility make it a widely used analytical technique.
Conclusion
In conclusion, High Performance Liquid Chromatography (HPLC) is a powerful analytical technique that allows for the separation and quantification of components in a sample.
By utilizing different types of HPLC, such as reverse-phase, normal-phase, ion-exchange, and size-exclusion, various applications in pharmaceutical analysis, environmental analysis, food analysis, forensic analysis, and more can be achieved.
While HPLC has its limitations, it remains an essential tool for accurate and precise analysis in various fields.

Erzsebet Frey (Eli Frey) is an ecologist and online entrepreneur with a Master of Science in Ecology from the University of Belgrade. Originally from Serbia, she has lived in Sri Lanka since 2017. Eli has worked internationally in countries like Oman, Brazil, Germany, and Sri Lanka. In 2018, she expanded into SEO and blogging, completing courses from UC Davis and Edinburgh. Eli has founded multiple websites focused on biology, ecology, environmental science, sustainable and simple living, and outdoor activities. She enjoys creating nature and simple living videos on YouTube and participates in speleology, diving, and hiking.