An antibiotic sensitivity test helps determine which antibiotics can effectively treat a bacterial infection. You’ll start by collecting a sample and preparing a pure bacterial culture. Next, you’ll grow the bacteria on a Mueller-Hinton agar plate and apply antibiotic disks. After incubation, you’ll observe and measure zones of inhibition around the disks. These measurements are compared to standardized breakpoints to interpret the results. You’ll then report the findings as susceptible, intermediate, or resistant to guide treatment decisions. Understanding this procedure is essential for healthcare professionals combating antibiotic resistance and ensuring ideal patient care. Dive deeper to master the nuances of this important diagnostic tool.
Sample Collection and Preparation
To begin the antibiotic sensitivity test, proper sample collection and preparation are vital. You’ll need to obtain a pure culture of the bacteria you’re testing. This typically involves collecting a clinical sample from the patient, such as blood, urine, or wound swabs. Confirm you’re using sterile techniques to avoid contamination.
Once you’ve collected the sample, you’ll need to isolate the bacteria. Streak the sample onto an appropriate agar plate and incubate it for 18-24 hours. After incubation, select a few well-isolated colonies and transfer them to a sterile broth medium. Incubate this broth culture for another 2-4 hours until it reaches a turbidity equivalent to a 0.5 McFarland standard.
Next, you’ll prepare the inoculum for the sensitivity test. Use a sterile swab to collect the bacteria from the broth culture. Remove excess liquid by pressing the swab against the tube’s inner wall. You’ll use this swab to inoculate the Mueller-Hinton agar plate, which is the standard medium for antibiotic sensitivity testing.
Streak the entire surface of the agar plate with the swab, rotating the plate 60 degrees between each streak to guarantee even distribution. Allow the plate to dry for about 5 minutes before applying antibiotic discs.
It’s essential to maintain sterile conditions throughout this process. Use a laminar flow hood if available, and always work near a flame. Label all plates and cultures clearly with the patient’s information and the date. By following these steps carefully, you’ll have a properly prepared sample ready for antibiotic sensitivity testing.
Bacterial Culture Growth
After preparing your inoculated Mueller-Hinton agar plate, the next step is to promote bacterial culture growth. This vital phase allows the bacteria to multiply, forming a visible lawn on the agar surface. You’ll need to incubate the plate under specific conditions to guarantee peak growth.
Place your inoculated plate in an incubator set to 35-37°C (95-98.6°F), which mimics the human body temperature and provides an ideal environment for most pathogenic bacteria. Make sure the plate is inverted to prevent condensation from dripping onto the agar surface, which could interfere with bacterial growth and subsequent test results.
The incubation time typically ranges from 16 to 24 hours, depending on the bacterial species you’re working with. During this period, the bacteria will reproduce and form colonies on the agar surface. It is vital to check your plate periodically to monitor growth progress.
You’ll know the bacterial culture has grown sufficiently when you observe a uniform, confluent lawn of growth across the agar surface. This lawn should be thick enough to be visible but not so dense that individual colonies are indistinguishable.
If you don’t see adequate growth after 24 hours, you may need to extend the incubation time. However, be cautious not to over-incubate, as this can lead to bacterial overgrowth and potentially skew your antibiotic sensitivity results.
Once you’ve achieved satisfactory bacterial growth, you can proceed to the next step in the antibiotic sensitivity test procedure. Remember, consistent and controlled growth is key to obtaining accurate and reliable results in your antibiotic sensitivity testing.
Antibiotic Disk Application
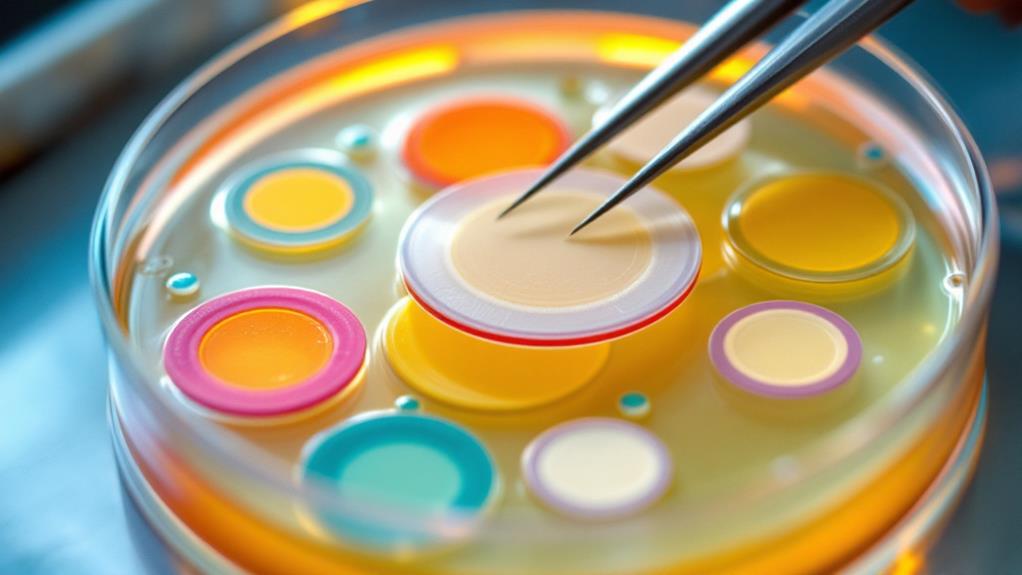
With your bacterial lawn ready, it’s time to apply the antibiotic disks. You’ll need a sterile forceps and a set of commercially prepared antibiotic disks. These disks are small, circular pieces of filter paper impregnated with specific concentrations of various antibiotics.
First, remove the lid of the agar plate carefully to avoid contamination. Using the sterile forceps, pick up an antibiotic disk by its edge. Place the disk gently onto the surface of the agar, ensuring good contact between the disk and the bacterial lawn. Press down lightly with the forceps to secure it in place.
Continue placing additional antibiotic disks on the same plate, spacing them evenly and at least 24 mm apart from each other and the edge of the plate. This spacing prevents overlapping zones of inhibition. Typically, you’ll place 4-6 disks per standard 100 mm agar plate.
As you work, label the bottom of the plate with the names or codes of the antibiotics you’ve applied. This step is essential for accurate result interpretation later. Once you’ve placed all the disks, replace the lid promptly.
Remember to flame-sterilize your forceps between handling different antibiotic disks to prevent cross-contamination. Work efficiently but carefully to minimize the time the plate is exposed to potential contaminants in the air.
After applying the disks, invert the plate and incubate it at the appropriate temperature for your bacterial species, usually 35-37°C for most pathogens. The incubation period typically lasts 16-24 hours, allowing sufficient time for bacterial growth and antibiotic diffusion.
Incubation and Observation
You’ve now reached a vital stage in the antibiotic sensitivity test: incubation and observation. After applying the antibiotic disks, you’ll need to incubate the inoculated plates to allow bacterial growth and antibiotic diffusion.
Place the prepared plates in an incubator set at 35-37°C for 16-18 hours. Confirm the plates are inverted to prevent condensation from dripping onto the agar surface. During this time, the bacteria will grow, and the antibiotics will diffuse into the agar, creating zones of inhibition around the disks where susceptible bacteria can’t grow.
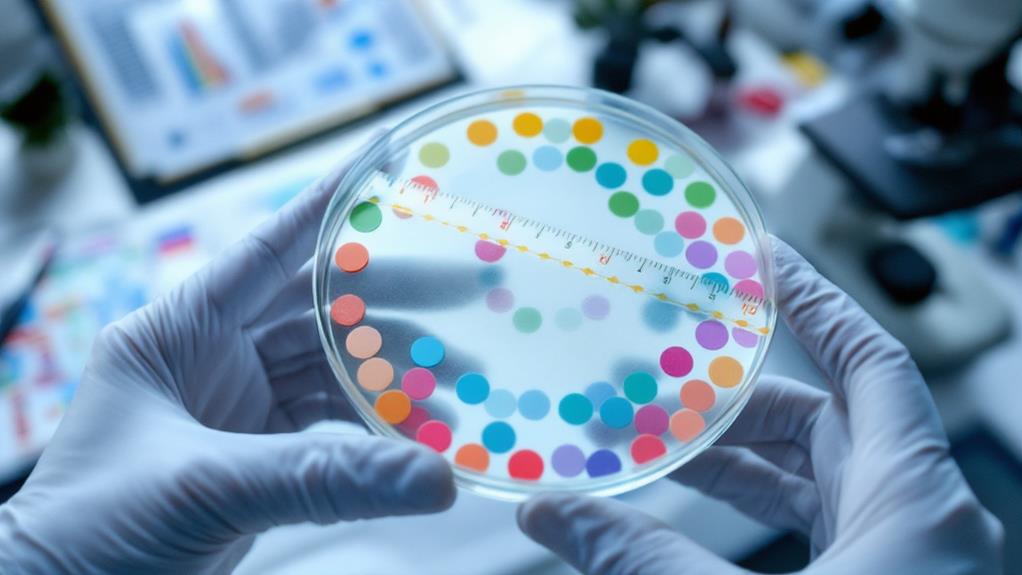
After incubation, you’ll observe and measure the zones of inhibition. Remove the plates from the incubator and examine them under good lighting. Look for clear circular areas around each antibiotic disk where bacterial growth is inhibited. Use a ruler or caliper to measure the diameter of each zone in millimeters, including the disk itself.
Record your measurements carefully, noting any irregularities or incomplete zones. Some fast-growing bacteria may require earlier readings, while slow-growing ones might need extended incubation. Always follow the standard guidelines for your specific test.
Interpret the results by comparing your measurements to established breakpoints for each antibiotic. These breakpoints categorize the bacterial strain as susceptible, intermediate, or resistant to each tested antibiotic. Remember that proper interpretation requires considering factors like the bacterial species, antibiotic concentration, and specific testing conditions.
Lastly, dispose of the plates according to your lab’s biosafety protocols. Proper documentation and reporting of results are essential for guiding appropriate antibiotic therapy and monitoring resistance trends.
Measuring Inhibition Zones
Measuring inhibition zones accurately is a key step in interpreting antibiotic sensitivity test results. After incubation, you’ll observe clear zones around the antibiotic discs where bacterial growth has been inhibited. To measure these zones, you’ll need a ruler or caliper, good lighting, and a dark background for contrast.
Start by placing your agar plate on a dark, non-reflective surface. Use a ruler or caliper to measure the diameter of each inhibition zone in millimeters. Measure from one edge of the clear zone to the opposite edge, including the diameter of the antibiotic disc. If the zone isn’t perfectly circular, take measurements from several angles and calculate the average.
When measuring, make certain you’re looking straight down at the plate to avoid parallax errors. Don’t remove the lid of the plate, as this could lead to contamination. If you’re using digital calipers, be careful not to touch the agar surface.
For each antibiotic, you’ll need to compare your measurements to standardized breakpoints. These breakpoints categorize the bacteria as susceptible, intermediate, or resistant to the antibiotic. You’ll find these standards in reference guides like the Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines.
Record your measurements and interpretations carefully. If you’re using automated systems, follow the manufacturer’s instructions for calibration and use. Some systems can measure zones and interpret results automatically, reducing human error.
Interpreting Test Results
Interpreting test results is a critical step in the antibiotic sensitivity testing process. After measuring the inhibition zones, you’ll need to compare these measurements to established standards to determine the susceptibility of the bacteria to each antibiotic.
You’ll typically use a reference chart provided by the disc manufacturer or your laboratory’s guidelines. This chart will list the antibiotics and their corresponding breakpoints for susceptible, intermediate, and resistant categories. Match your measured zone diameters to these breakpoints.
If the zone diameter is equal to or larger than the susceptible breakpoint, you’ll classify the bacteria as susceptible to that antibiotic. This means the antibiotic is likely to be effective in treating the infection. If the zone is smaller than the resistant breakpoint, you’ll categorize the bacteria as resistant. In this case, the antibiotic won’t be effective. Measurements falling between these two breakpoints indicate intermediate susceptibility.
Remember that these interpretations aren’t absolute. They’re based on standardized in vitro conditions and may not perfectly reflect in vivo efficacy. Clinical factors, such as the site of infection and patient characteristics, should also be considered when selecting treatment.
You’ll need to report your results clearly, typically using the categories “S” for susceptible, “I” for intermediate, and “R” for resistant. Some labs may require you to include the actual zone diameters alongside these interpretations.
Lastly, always look for patterns in your results. Multiple drug resistance or unexpected susceptibilities may indicate the need for further testing or consultation with a microbiologist or infectious disease specialist.
Reporting and Treatment Recommendations
After interpreting the antibiotic sensitivity test results, it’s time to report your findings and provide treatment recommendations. You’ll need to clearly communicate the results to the healthcare provider or clinician responsible for the patient’s care. Start by summarizing the bacterial isolate identified and its susceptibility pattern to the tested antibiotics.
In your report, include the following information:
- Patient details and specimen type
- Bacterial species identified
- List of antibiotics tested
- Susceptibility results (sensitive, intermediate, or resistant)
- Minimum Inhibitory Concentration (MIC) values, if applicable
When recommending treatment options, consider the following factors:
- The site of infection
- Patient’s medical history and allergies
- Local antibiotic resistance patterns
- Drug availability and cost
- Potential side effects and drug interactions
Suggest first-line antibiotics that show sensitivity to the identified pathogen. If multiple options are available, prioritize narrow-spectrum antibiotics to reduce the risk of developing resistance. Include alternative treatments in case of allergies or contraindications.
Provide guidance on the appropriate dosage, route of administration, and duration of treatment based on current clinical guidelines. If the pathogen shows resistance to multiple antibiotics, you may need to recommend combination therapy or consult with an infectious disease specialist.
Remember to emphasize the importance of completing the full course of antibiotics to prevent the development of resistance. If applicable, suggest follow-up cultures to monitor treatment efficacy. By providing extensive and clear recommendations, you’ll help guarantee ideal patient care and antibiotic stewardship.

Erzsebet Frey (Eli Frey) is an ecologist and online entrepreneur with a Master of Science in Ecology from the University of Belgrade. Originally from Serbia, she has lived in Sri Lanka since 2017. Eli has worked internationally in countries like Oman, Brazil, Germany, and Sri Lanka. In 2018, she expanded into SEO and blogging, completing courses from UC Davis and Edinburgh. Eli has founded multiple websites focused on biology, ecology, environmental science, sustainable and simple living, and outdoor activities. She enjoys creating nature and simple living videos on YouTube and participates in speleology, diving, and hiking.

