Nitrogen fixation is the process that converts atmospheric nitrogen into forms usable by living organisms. You’ll encounter two main types: natural and artificial. In nature, bacteria in soil and plant roots, as well as lightning, perform this task. Industrially, the Haber-Bosch process creates ammonia for fertilizers. While essential for agriculture and ecosystems, excessive nitrogen can harm the environment through pollution and greenhouse gas emissions. Sustainable practices aim to balance nitrogen use and minimize negative impacts. Understanding this process is imperative for food security, environmental protection, and addressing climate change. There’s much more to uncover about this fascinating and essential cycle.
Natural Nitrogen Fixation Methods

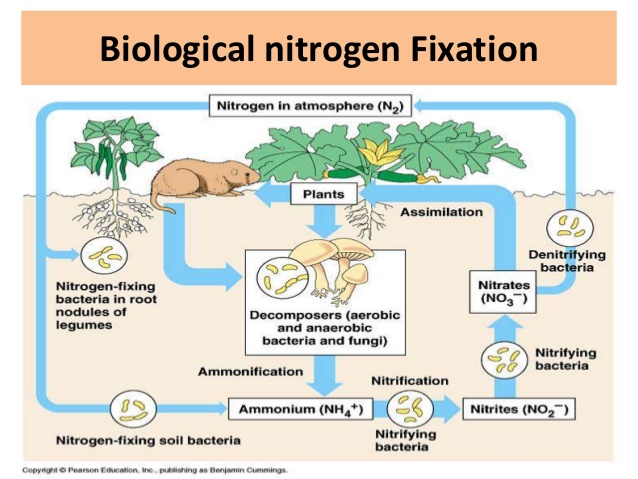
Natural nitrogen fixation methods are essential processes that convert atmospheric nitrogen into forms usable by plants and other organisms. You’ll find that these methods occur without human intervention, playing a vital role in the global nitrogen cycle.
The most common natural method is biological nitrogen fixation. This process is carried out by certain bacteria and archaea called diazotrophs. You’ll often find these microorganisms living symbiotically in the root nodules of legumes, such as peas, beans, and clover. They use an enzyme called nitrogenase to break the strong triple bond of atmospheric nitrogen (N2) and combine it with hydrogen to form ammonia (NH3).
Lightning is another natural nitrogen fixation method you should be aware of. When lightning strikes, it generates enough heat and pressure to break nitrogen molecules in the air, allowing them to combine with oxygen and form nitrogen oxides. These compounds then dissolve in rainwater, creating nitrates that plants can absorb from the soil.
You’ll also encounter nitrogen fixation in aquatic environments. Some cyanobacteria, also known as blue-green algae, can fix nitrogen in water bodies. This process is particularly important in marine ecosystems, where it supports the growth of phytoplankton and other organisms at the base of the food chain.
Understanding these natural nitrogen fixation methods is essential for maintaining ecosystem balance and developing sustainable agricultural practices. By harnessing these processes, you can reduce reliance on synthetic fertilizers and promote more environmentally friendly food production methods.
Biological Nitrogen-Fixing Organisms
Diversity characterizes the world of biological nitrogen-fixing organisms. You’ll find these remarkable creatures in various ecosystems, from soil to water, playing an essential role in the nitrogen cycle. These organisms can convert atmospheric nitrogen (N₂) into ammonia (NH₃), a form that plants can use.
Bacteria are the most common nitrogen fixers. You’ll encounter free-living bacteria like Azotobacter and Clostridium in soil, while aquatic environments host cyanobacteria such as Anabaena and Nostoc. These microorganisms use the enzyme nitrogenase to break the strong triple bond of N₂.
Symbiotic relationships between bacteria and plants are another fascinating aspect of biological nitrogen fixation. You’ll see this in action with Rhizobium bacteria, which form nodules on the roots of legumes like peas, beans, and clover. These bacteria provide the plant with fixed nitrogen, while the plant offers carbohydrates and a protected environment.
Some plants have evolved to house nitrogen-fixing bacteria within their tissues. You’ll find this in certain trees like alders and some tropical species. These plants have specialized structures called actinorhizal nodules that host Frankia bacteria.
In marine environments, you’ll discover nitrogen fixation occurring in coral reefs and seagrass beds. Here, cyanobacteria play a significant role in providing fixed nitrogen to these nutrient-limited ecosystems.
Understanding these biological nitrogen-fixing organisms is imperative for agriculture and ecosystem management. They offer sustainable alternatives to synthetic fertilizers and play a key role in maintaining soil fertility and ecosystem health.
Chemical Nitrogen Fixation Processes
Chemical nitrogen fixation processes stand in stark contrast to their biological counterparts. While nature relies on specialized organisms to convert atmospheric nitrogen into usable forms, industrial methods employ high-temperature, high-pressure reactions to achieve the same goal.
The most significant chemical process for nitrogen fixation is the Haber-Bosch process. Developed in the early 20th century, it’s still widely used today to produce ammonia, an essential component in fertilizers. In this process, you’ll find nitrogen gas and hydrogen gas reacting under extreme conditions: temperatures around 450°C and pressures up to 200 atmospheres. An iron catalyst facilitates the reaction, helping to break the strong triple bond of the nitrogen molecule.
You’ll also encounter other chemical methods for nitrogen fixation. The Birkeland-Eyde process, for instance, uses an electric arc to oxidize nitrogen from the air, forming nitrogen oxides. These are then absorbed in water to produce nitric acid. While less efficient than the Haber-Bosch process, it’s still used in some applications.
Another method you might come across is the cyanamide process. It involves passing nitrogen gas over heated calcium carbide, resulting in calcium cyanamide. When hydrolyzed, this compound releases ammonia.
These chemical processes have revolutionized agriculture by providing a steady supply of nitrogen-based fertilizers. However, they’re energy-intensive and often rely on fossil fuels, leading to significant carbon emissions. As you explore this topic further, you’ll find ongoing research into more sustainable chemical nitrogen fixation methods, aiming to reduce environmental impact while meeting global fertilizer demands.
Industrial Nitrogen Fixation Applications
Industrial applications of nitrogen fixation extend far beyond the production of fertilizers. You’ll find that this process plays a significant role in various sectors, including manufacturing, pharmaceuticals, and energy production.
In the chemical industry, you’ll see nitrogen fixation used to create ammonia, a key component in the production of nitric acid, urea, and other nitrogen-based compounds. These compounds are fundamental for manufacturing plastics, explosives, and synthetic fibers. You’ll also encounter nitrogen fixation in the production of melamine, a versatile material used in laminates, adhesives, and flame retardants.
When you look at the pharmaceutical industry, you’ll notice that nitrogen fixation is important for producing many medications. It’s used to synthesize amino acids, which are the building blocks of proteins and essential for drug development. You’ll find nitrogen-containing compounds in antibiotics, painkillers, and various other pharmaceuticals.
In the energy sector, you’ll observe nitrogen fixation’s role in the production of hydrogen cyanide, a compound used in the extraction of gold and silver from ores. It’s also employed in the manufacture of sodium cyanide, which is used in electroplating processes.
You’ll discover that nitrogen fixation is fundamental in the production of cleaning agents, such as detergents and disinfectants. It’s also used in the creation of dyes and pigments for textiles and paints.
In the food industry, you’ll encounter nitrogen fixation in the production of food preservatives and flavor enhancers. It’s also used to create packaging materials that help extend the shelf life of various products.
Environmental Impact of Nitrogen Fixation
While nitrogen fixation has revolutionized agriculture and industry, it is crucial to evaluate its environmental implications. The increased availability of reactive nitrogen through artificial fixation has led to significant ecological consequences. You’ll find that excess nitrogen in ecosystems can cause eutrophication in water bodies, leading to algal blooms and oxygen depletion. This process harms aquatic life and disrupts entire food chains.
In terrestrial ecosystems, you’ll notice that excessive nitrogen can alter plant communities. It often favors fast-growing species, potentially reducing biodiversity. You might observe changes in soil chemistry, including acidification, which can affect plant growth and microbial communities. The runoff of nitrogen-rich fertilizers into water systems contributes to the formation of “dead zones” in coastal areas, where marine life struggles to survive.
You should also consider the atmospheric impact. The production of nitrogen-based fertilizers through the Haber-Bosch process consumes vast amounts of energy, primarily from fossil fuels. This contributes to greenhouse gas emissions and climate change. Additionally, you’ll find that some fixed nitrogen is converted to nitrous oxide, a potent greenhouse gas, through soil processes.
To mitigate these impacts, you can adopt precision agriculture techniques, optimizing fertilizer use. You might also explore alternative nitrogen sources, such as biological nitrogen fixation or recycled organic matter. By understanding and addressing the environmental consequences of nitrogen fixation, you can work towards more sustainable agricultural and industrial practices.

Erzsebet Frey (Eli Frey) is an ecologist and online entrepreneur with a Master of Science in Ecology from the University of Belgrade. Originally from Serbia, she has lived in Sri Lanka since 2017. Eli has worked internationally in countries like Oman, Brazil, Germany, and Sri Lanka. In 2018, she expanded into SEO and blogging, completing courses from UC Davis and Edinburgh. Eli has founded multiple websites focused on biology, ecology, environmental science, sustainable and simple living, and outdoor activities. She enjoys creating nature and simple living videos on YouTube and participates in speleology, diving, and hiking.

